Abstract
Intro: Acute myeloid leukemia (AML) is a heterogeneous clonal hematopoietic neoplasm. The cytogenetic changes associated with AML impact response rate and survival, and is one of the most important independent prognostic factors. AML with inv(3)(q21.3q26.2) or; GATA2, MECOMaccounts for 1-2% of all forms of AML. This form is associated with a younger age at diagnosis, poor response to standard induction chemotherapy, and very poor long-term prognosis with an overall survival of <10%. Unfortunately, traditional intensive induction chemotherapy has a reported remission rates of 10-20% (Raya et al. Hematology 2015;20(8):435-441). Clearly, alternative chemotherapy approaches are needed to effectively treat AML with inv(3). We previously presented a case report of a patient with inv(3) receiving induction chemotherapy with a combination of a hypomethylating agent (HMA) with lenalidomide and achieving remission (Platzbecker, et al. Leukemia 2013;27:1813-1819, Foucar et al. American Journal of Clinical Pathology2015; 144(1): 6-18). To further address the effectiveness of this regimen, we performed a retrospective cohort study comparing outcomes with HMA plus lenalidomide to standard intensive induction therapies in newly diagnosed and relapsed/refractory AML with inv(3).
Methods: We conducted a single-center, IRB-approved, retrospective cohort analysis of 939 patients who received therapy for AML at the University of Michigan between March 2005-June 2018. 15/939 (1.6%) patients tested positive for inv(3)(q21.3q26.2), and they were divided into two cohorts: a lenalidomide-based regimen or other chemotherapy induction approaches. Data abstraction of patient, disease and treatment-related variables was performed through manual chart review. The primary outcome was overall response rate (ORR), reported as the combination of complete remission (CR) and complete remission with incomplete count recovery (CRi). Secondary endpoints included overall survival (OS), 30- and 60-day mortality, event free survival (EFS), and duration of response. All data were analyzed using SPSS software, version 24.0 (SPSS, Inc., Chicago, IL).
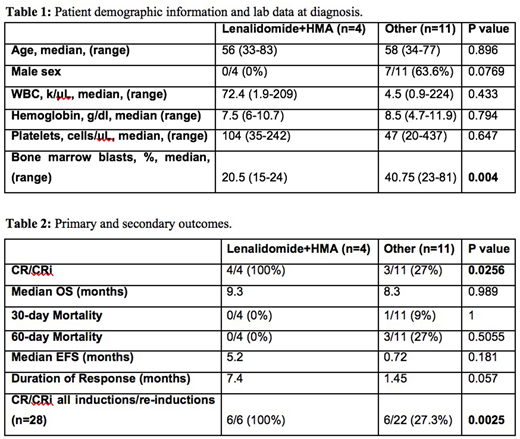
Results: In total, 15 patients were positive for inv(3)(q21.3q26.2) and underwent treatment at the University of Michigan. 4/16 (25%) received lenalidomide and HMA as primary therapy. Patient demographics and lab values at diagnosis are shown in Table 1. 4/4 (100%) of patients receiving lenalidomide with HMA as first line therapy achieved CR/CRi while 3/11 (27.3%) of patients receiving other chemotherapeutic agents initially achieved CR/CRi (p=0.0256). Duration of response was numerically longer in patients receiving lenalidomide-based therapies (7.4 months vs. 1.45 months; p=0.057). Primary and secondary outcomes in patients receiving lenalidomide plus HMA and other chemotherapies are shown in Table 2. 2/13 patients requiring salvage therapy received lenalidomide with HMA. Both of these patients achieved CR/CRi, while 3/11 (27.3%) of patients receiving other chemotherapy achieved CR/CRi. Combining initial and salvage inductions, 6/6 (100%) achieved CR/CRi with lenalidomide with HMA versus 6/22 (27.3%) with other chemotherapy (p=0.0025).
Discussion: AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2, MECOMis notorious for a poor response to standard induction chemotherapy and a reported remission rate of 10-20% with traditional chemotherapy (Raya et al. Hematology 2015;20(8):435-441).In this cohort, all patients who received lenalidomide with or without a HMA achieved CR/CRi compared to 27% of patients receiving other chemotherapy. Importantly, the median duration of response with lenalidomide and HMA was longer than traditional chemotherapy, although not statistically significant, likely due to the small sample size. The high ORR and reasonable duration of response could allow for potentially curative alloHCT in these high-risk AML patients. Our study is limited by the small sample size due to the rarity of this AML subtype, but the initial data suggests that lenalidomide plus HMA is a promising approach for patients with AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2, MECOM. A multicenter, prospective trial should be considered to compare the efficacy of traditional cytotoxic chemotherapy approaches versus lenalidomide plus HMA to improve outcomes in this subtype of AML.
Bixby:GlycoMimetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal